 2020/07/07
2020/07/07ACE COVID-19 IgG / IgM Dual Detection Kit
The ACE COVID-19 IgG / IgM Dual Detection Kit provides information about the COVID-19 antibodies in human serum or plasma.
Testing Principles
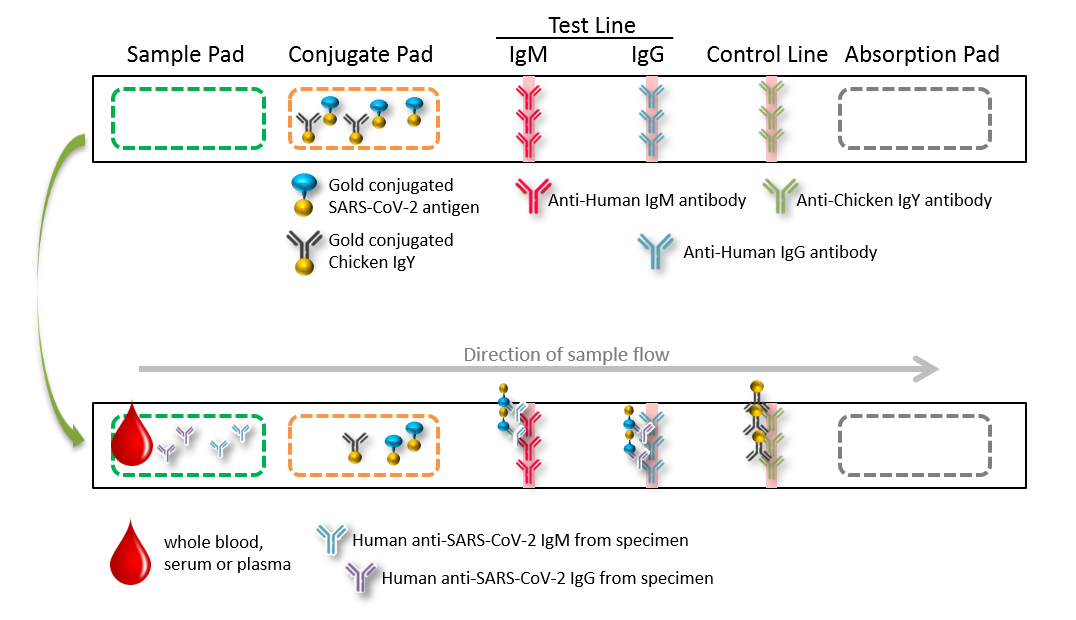
The ACE COVID-19 IgG / IgM Dual Detection Kit is a lateral flow chromatographic immunoassay which can detect antibodies against the SARS-CoV-2 virus. The test card consists of:
1) Binding pad: colloidal gold-labeled recombinant SARS-CoV-2 (2019-nCoV) antigens and quality control antibody;
2) NC membrane: coated with two Test Lines (G and M lines) and a quality Control line (C line). M-line coated with mouse anti-human IgM monoclonal antibody for detection of SARS-CoV-2 IgM antibodies; G-line coated with mouse anti-human IgG monoclonal antibody for detection of SARS-CoV-2 IgG antibodies; C-line coating with quality control antibody.
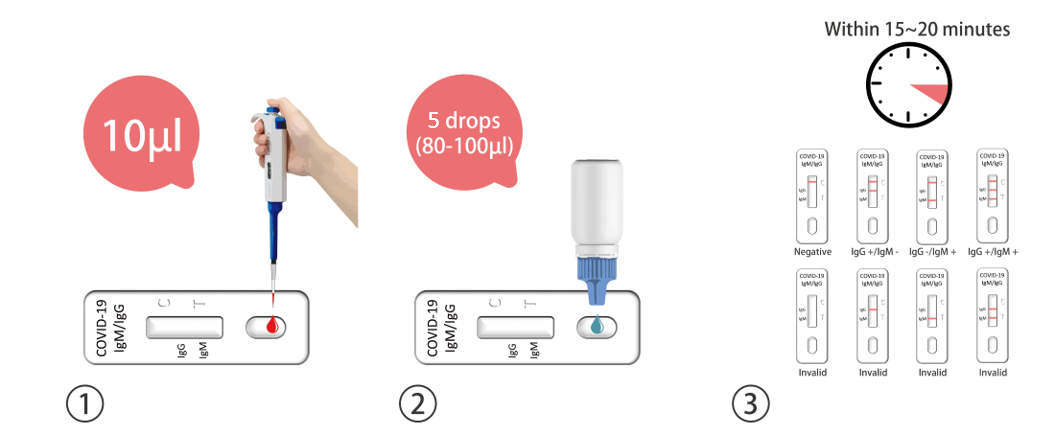
Testing Procedure
Performance - Clinical Studies
|
|
PCR Comparator* |
Total |
||
|
Pos |
Neg |
|||
|
ACE COVID-19 Dual Antibody Detection Kit |
Pos |
26 |
0 |
26 |
|
Neg |
1 |
51 |
52 |
|
|
Total |
27 |
51 |
78 |
|
*Note: Serum and plasma samples were collected from the same patients for serology testing between 1 day and > 45 days after PCR sample collection.
Positive Percent Agreement (PPA)= (Antibody positive)/(PCR positive)
PPA: 96.80% (26/27)
Negative Percent Agreement: (NPA) = (Antibody negative)/(PCR negative)
NPA: 100.00% (51/51)
Performance - Cross-Reactivity
|
Cross-reactivity |
High-risk groups |
Total |
||||
|
Medical staff1 |
Suspect symptoms2 |
|||||
|
Serum |
Plasma |
Serum |
Plasma |
|||
|
ACE COVID-19 Dual Antibody Detection Kit |
POS |
0 |
0 |
0 |
0 |
0 |
|
NEG |
69 |
0 |
57 |
6 |
132 |
|
|
Total |
69 |
63 |
132 |
|||
Contact us for more information
With the now global outbreak of COVID-19, SARS-CoV-2 early diagnostics and infection prevention and control has become critical. ACE Biolabs can provide COVID-19 Total Solutions for Detection and Surveillance :

.png)