.jpg)
Coronavirus Protein Microarray
Features
Highly sensitive - Can detect 50 pg antibodies which is 1000x lower than ELISA method.
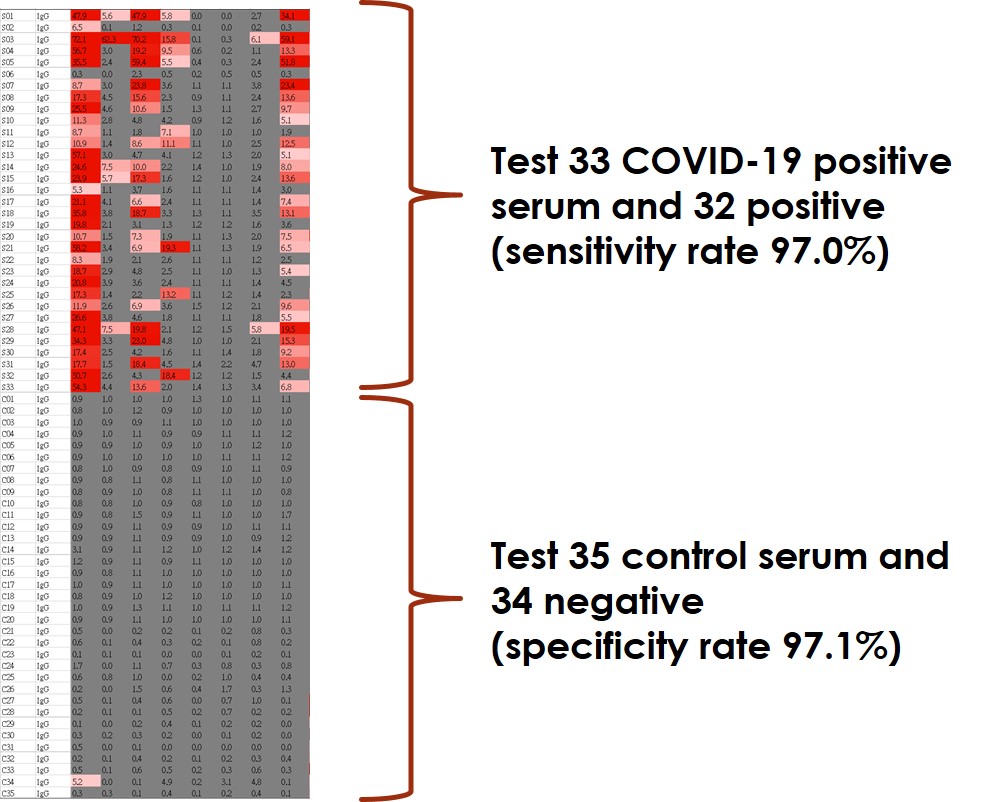
Test ~30 COVID-19 positive serum with sensitivity rate 97% - higher than FDA request 90%
Test ~30 control serum with specificity rate 97% - higher than FDA request 95%
Find a panel of biomarkers for rapid test - 2-3 biomarkers with same sensitivity and specificity
Experimental procedures for the serological antibodies
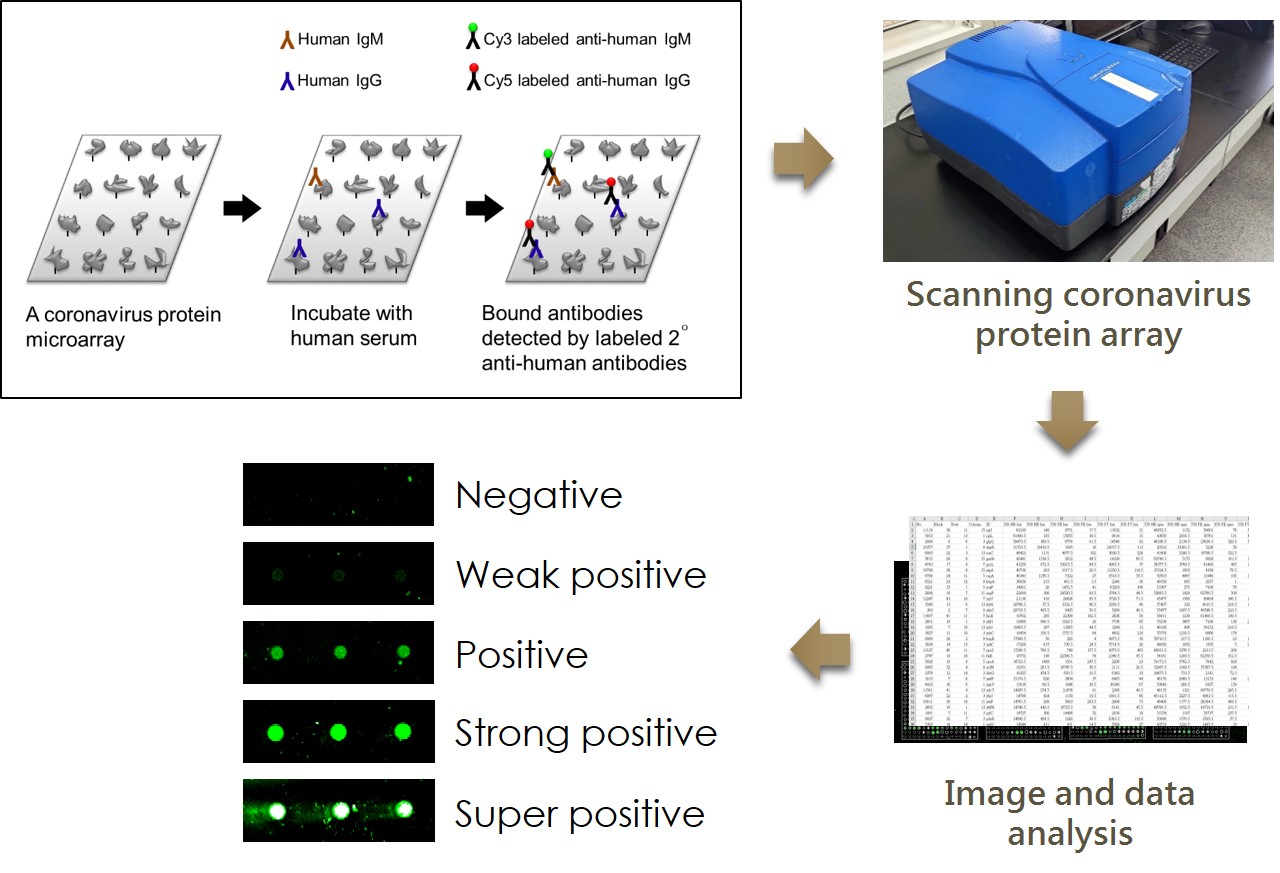
Quality control of the SARS-CoV-2 Microarray
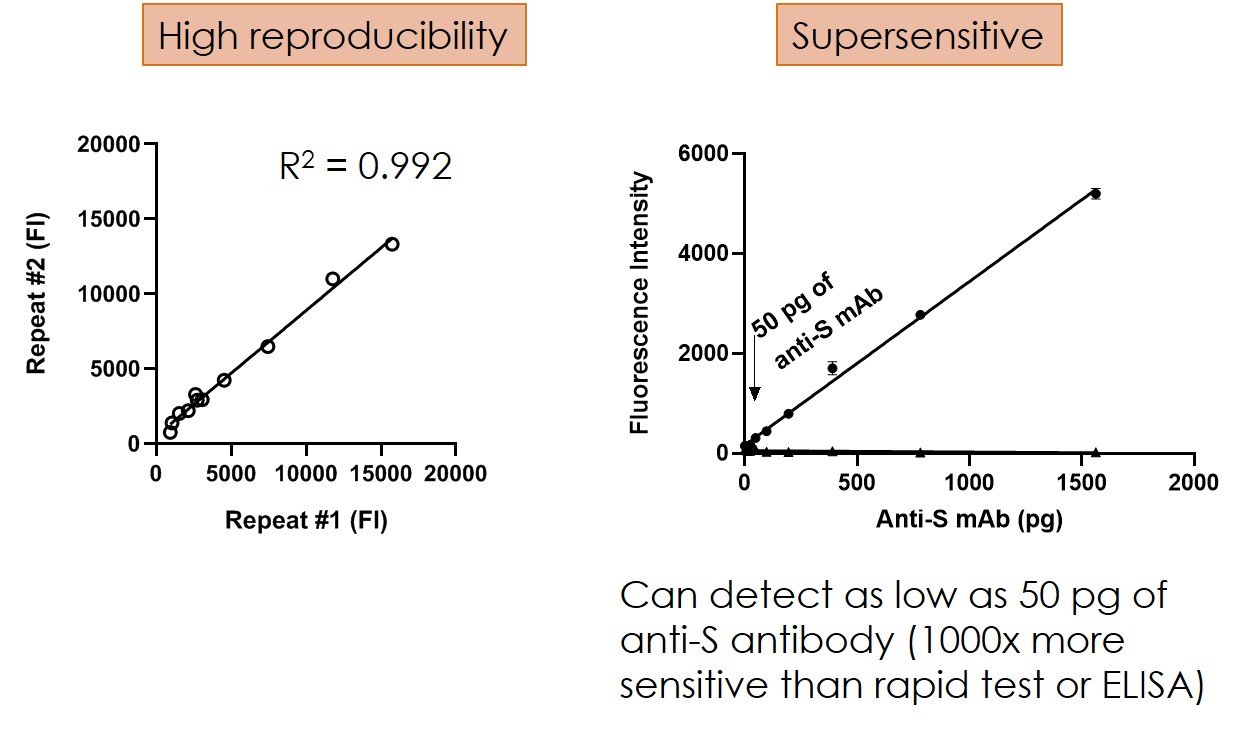
Technical advances
Multiplexed
Simultaneously detect serum IgG/IgM and profile the immune responses against many coronaviral proteins.
Accurate
Since we compare many proteins from SARS-CoV-2, SARS-CoV, and MERS-CoV, the false negative or non-specific bindings can be evaluated. Also, the combination of multiple markers always can help the specificity and accuracy for the diagnosis.
Flexible
We can include the protein with common mutations that observed in the clinical samples and provide a deeper view of the impact of SARS-CoV-2 mutations. For the clinical applications, it is also flexible to discover and combine different markers for the diagnosis, prognosis, and severity classifications.
Sensitive
Only need 1 microliter serum the assay which is less than a drop of blood. In a microliter, we can acquire numerous data information for the research, clinical, and epidemical uses.
Specific
The protein microarray is a useful platform to show the specificity of the biologicals. The coronavirus protein array will speed up the process of developing biologicals and help to evaluate the outcome of humoral immunity for the vaccines.

.png)