Introduction
Trisodium citrate dihydrate is a conjμgate base of a weak acid, can perform as a biological buffering agent because it resists changes in pH. Citric acid is one of a series of compounds responsible for the physiological oxidation of fats, carbohydrates and proteins to carbon dioxide and water. Sodium Citrate, Dihydrate is often used to prepare sodium citrate buffer for antigen retrieval of tissue samples. The citrate solution is designed to break protein cross-links; thus, unmasking antigens and epitopes in formalin-fixed and paraffin embedded tissue sections, resulting in enhancing staining intensity of antibodies. Citrate has anticoagulant activity and as a calcium chelator, it forms complexes that disrupt the tendency of blood to clot.An anticoagulant also used as a biological buffer.
General Information
| Application | Chemical |
|---|---|
| Cas No. | 6132-04-3 |
| Purity | 99% |
| Molecular Weight | 294.1 |
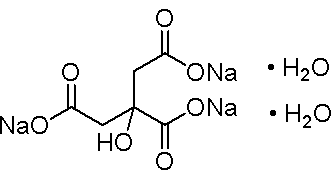
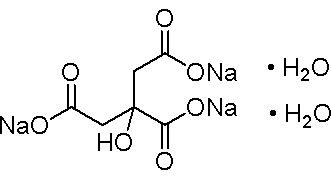
| Formula | C6H5Na3O7·2H2O |
| Solubility | Soluble in water (29.4 g/l) at 20 °C. Insoluble in ethanol |
| Storage instruction | Store at room temperature |
| Alias | Citric acid trisodium salt dihydrate |





.png)