Trypsin powder from porcine pancreas (1:250)
- Catalog Number : CC1014
- Number : CC1014-s
-
Size:
Qty : - Price : Request Inquiry
Introduction
The typical use for trypsin is in removing adherent cells from a culture surface.
This product is a trypsin freeze-dried powder derived from porcine pancreas. The virus is inactivated by γ-ray treatment, and the enzyme activity is> 250 units/mg. It is widely used for cell passage. The adherent cells are digested and hydrolyzed from the culture plate to prepare a single cell suspension. The commonly used working concentration is 0.25-5% (w/v).
Trypsin is a serine protease commonly found in the digestive system of vertebrates. It hydrolyzes proteins and is secreted in the pancreas in the form of inactive trypsinogen. The cleavage site of the peptide chain is mainly located at the carboxyl end of lysine or arginine (except when the two are immediately adjacent to proline). Trypsin consists of two subunits, α subunit (consisting of 2 polypeptide chains) and β subunit (consisting of 1 polypeptide chain). Its hydrolysis activity can be inhibited by a variety of inhibitors, including: 1) organophosphorus compounds, such as isopropyl fluorophosphate; 2) natural trypsin inhibitors derived from pancreas, soybeans, green beans, and egg white; 3) silver ions; 4 ) Specific protease inhibitors, such as AEBSF, anti-protease, aprotinin, DFP, leupeptin, PMSF, TLCK, etc.
This product is a trypsin freeze-dried powder derived from porcine pancreas. The virus is inactivated by γ-ray treatment, and the enzyme activity is> 250 units/mg. It is widely used for cell passage. The adherent cells are digested and hydrolyzed from the culture plate to prepare a single cell suspension. The commonly used working concentration is 0.25-5% (w/v).
Trypsin is a serine protease commonly found in the digestive system of vertebrates. It hydrolyzes proteins and is secreted in the pancreas in the form of inactive trypsinogen. The cleavage site of the peptide chain is mainly located at the carboxyl end of lysine or arginine (except when the two are immediately adjacent to proline). Trypsin consists of two subunits, α subunit (consisting of 2 polypeptide chains) and β subunit (consisting of 1 polypeptide chain). Its hydrolysis activity can be inhibited by a variety of inhibitors, including: 1) organophosphorus compounds, such as isopropyl fluorophosphate; 2) natural trypsin inhibitors derived from pancreas, soybeans, green beans, and egg white; 3) silver ions; 4 ) Specific protease inhibitors, such as AEBSF, anti-protease, aprotinin, DFP, leupeptin, PMSF, TLCK, etc.
General Information
| Application | Cell |
|---|---|
| Cas No. | 9002-07-7 |
| Molecular Weight | 23.8 kDa |
| Solubility | Soluble in water, almost insoluble in ethanol and glycerin |
| Storage instruction | Store at 2 to 8°C |
| Alias | Trypsin, porcine pancreas; Parenzyme; Tryptar; Trypure; Parenzymol; U-4858 |
|
Apperance |
White to off-white powder |
|---|---|
|
Extinction coefficient |
E1%280=14.3 |
|
Isoelectric Point |
pH 10.5 |
|---|---|
|
Specific activity |
>250 U/mg |
* Unit definition :The activity causing a change in absorbance of 0.003/minute at 253 nm.
The definition and conversion of activity frequently encountered in trypsin freeze-dried powder products,
- 1 BAEE U: Under the conditions of 25°C and pH 7.6, with BAEE as the substrate, in a 3.2 mL reaction system, the absorbance value of A253 changes by 0.001 per minute, which is a trypsin activity unit.
- 1 TAME U: Under the condition of 25℃, pH 8.2, 0.001M Ca2+, the amount of sample that hydrolyzes 1 micromol TAME per minute.
- 1 USP U: Under the specified conditions, the sample activity that causes the absorbance value to change by 0.003 per minute.
- 1 TAME unit = 19.2 USP or NF units = 57.5 BAEE Unit





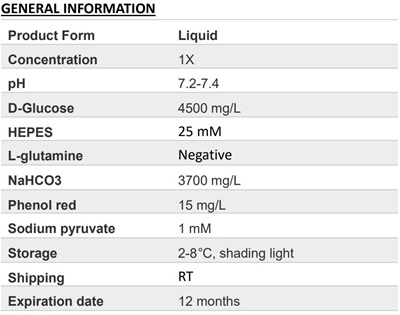
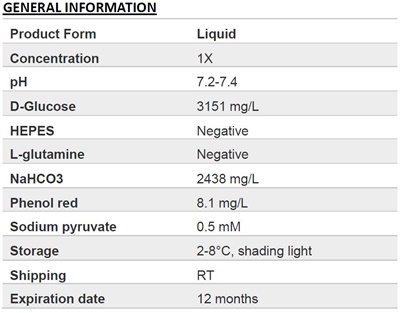


.png)