anti-CTLA-4 (Ipilimumab)
- Catalog Number : A510020
- Number : A510020-1mg
-
Size:
Qty : - Price : Request Inquiry
Introduction
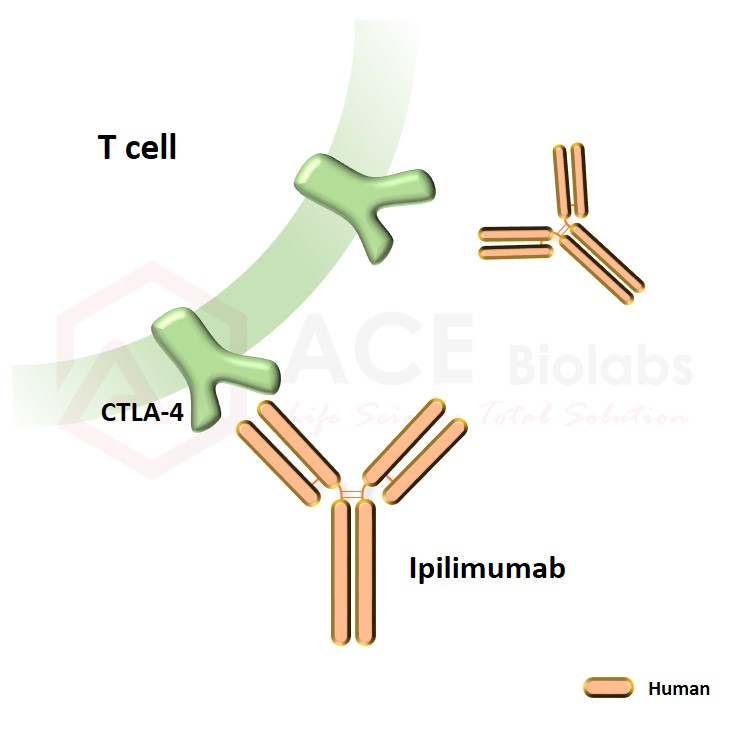
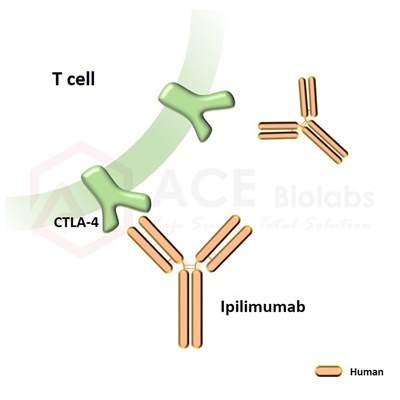
Ipilimumab biosimilar is a recombinant, human monoclonal antibody that binds to the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4). CTLA4 is an inhibitory receptor found at the surface of T-cells responsible for the downregulation of the immune response. Upon binding to CTLA4, ipilimumab acts as an immune checkpoint inhibitor and promotes T-cell activation against cancer cells. Ipilimumab was first approved by the FDA in 2011 for the treatment of melanoma and is now used to treat colorectal cancer, hepatocellular carcinoma, advanced melanoma, and renal cell carcinoma. It is currently under investigation for other types of advanced and metastatic cancers.
General Information
| Reactivity | Human |
|---|---|
| Application | Functional Assay |
| Host | CHO cells |
| Clonality | Monoclonal |
| Conjugate | Non-conjugated |
| Isotype | Human IgG1 |
| Cas No. | 477202-00-9 |
| Purity | >95%; < 0.75 EU/mg as determined by the LAL method |
| Molecular Weight | 148 kDa |
| Storage buffer | 0.01 M phosphate buffered saline (PBS) pH 7.2 |
| Storage instruction | This solution is stable for at least 4 weeks when stored at 2-8°C. For long term storage aseptically aliquot and store at –80°C.Avoid repeated freeze/thaw cycles. |
| Alias | MDX-010, BMS-734016, Yervoy |







.png)